Topic: The Atmosphere
The Atmosphere
The accumulation of water vapor, carbon dioxide, and nitrogen in Earth’s early atmosphere approximately 4 billion years ago resulted mainly from
(1) outgassing from Earth’s interior
(2) radioactive decay
(3) photosynthesis by the earliest land plants
(4) convection currents in Earth’s outer core
Earth’s early atmosphere contained carbon dioxide, sulfur dioxide, hydrogen, nitrogen, water vapor, methane, and ammonia. These gases were present in the atmosphere primarily because
(1) radioactive decay products produced in Earth’s core were released from Earth’s surface
(2) evolving Earth life-forms produced these gases through their activity
(3) Earth’s growing gravitational field attracted these gases from space
(4) volcanic eruptions on Earth’s surface released these gases from the interior
Outgassing of water vapor, carbon dioxide, and nitrogen initially formed Earth’s early
(1) lithosphere
(2) hydrosphere
(3) asthenosphere
(4) atmosphere
The major source of oxygen in Earth’s Early Proterozoic atmosphere is inferred to have been produced by
(1) oceanic cyanobacteria
(2) outgassing from volcanic eruptions
(3) radioactive decay in Earth’s inner core
(4) evaporation of ocean water
Which gas is inferred to have been absent from Earth’s atmosphere during the Early Archean Era?
(1) carbon dioxide
(2) nitrogen
(3) oxygen
(4) water vapor
Most of the oceanic oxygen that began to enter Earth’s atmosphere in the early Proterozoic Era was probably produced by
(1) formation of silicate rocks
(2) photosynthesis by cyanobacteria
(3) impact events on Earth’s surface
(4) outgassing from volcanoes
Scientists infer that oxygen first began to enter Earth’s atmosphere after the appearance of
(1) the earliest grasses
(2) the earliest flowering plants
(3) coal-forming forests
(4) oceanic cyanobacteria
Approximately 2.2 billion years ago, which gas was first added in large amounts to Earth’s atmosphere from life-forms that evolved in the oceans?
(1) carbon dioxide
(2) water vapor
(3) oxygen
(4) nitrogen
Scientists infer that oxygen in Earth’s atmosphere did not exist in large quantities until after
(1) the first multicellular, soft-bodied marine organisms appeared on Earth
(2) the initial opening of the Atlantic Ocean
(3) the first sexually reproducing organisms appeared on Earth
(4) photosynthetic cyanobacteria evolved in Earth’s oceans
Oxygen in Earth’s early atmosphere was first produced during the Precambrian from
(1) cyanobacteria in Earth’s oceans
(2) volcanic activity along plate boundaries
(3) the absorption of sunlight by plants
(4) evaporation of ocean water
In which two Earth regions is oxygen the second most abundant element by volume?
(1) crust and hydrosphere
(2) hydrosphere and troposphere
(3) troposphere and core
(4) core and crust
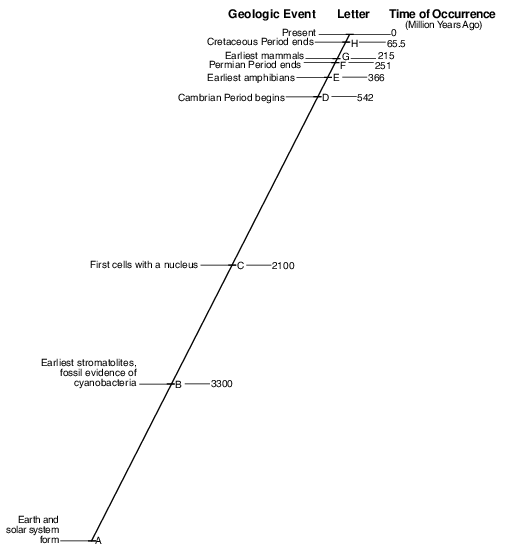
Describe the major change in Earth’s atmosphere that was occurring at the time when the first cells with a nucleus appeared on Earth. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — Oceanic oxygen began to enter the atmosphere.
• — Excess oxygen in the oceans escaped into the atmosphere.
• — A buildup of oxygen began.
• — Photosynthetic bacteria released oxygen.
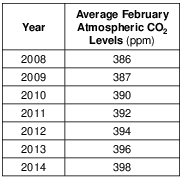
These measurements of atmospheric carbon dioxide were collected at an altitude of 3.4 kilometers. Identify the temperature zone of the atmosphere where these data were collected. [1]
Allow 1 credit for troposphere.
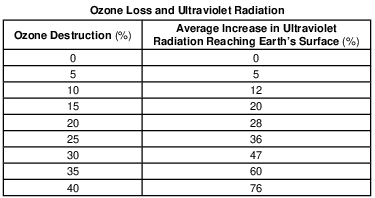
The ozone layer is mostly concentrated between 20 and 25 kilometers above Earth’s surface. State the name of the atmospheric temperature zone layer where this ozone concentration can be found. [1]
Allow 1 credit for stratosphere.
CFCs and Ozone
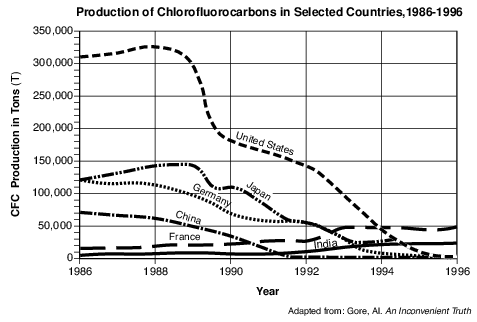
CFCs (chlorofluorocarbons) are chemicals that threaten to destroy stratospheric ozone. CFCs were first manufactured in 1928 to be used as chilling agents in refrigerators. In later years, they were used for cleaning electrical circuit boards and to make foam for insulation. Unfortunately, scientists found that these chemicals escaped into the atmosphere and rose to the stratosphere. In the stratosphere, intense ultraviolet (UV) radiation broke the CFCs down, producing chlorine, a gas that reacts with and destroys ozone. In 1974, two scientists identified the depletion of stratospheric ozone from the release of CFCs. After this discovery, 27 countries agreed to reduce production of CFCs, because ozone in the stratosphere protects all life from the Sun’s most damaging UV rays.
Write the chemical symbol for the element produced by the breakdown of CFCs. Describe one environmental impact that results from this element being released into Earth’s stratosphere. [1]
Chemical symbol: Environmental impact:
Allow 1 credit for the symbol Cl and a correct environmental impact. Acceptable responses include, but are not limited to:
• — reacts with and destroys ozone
• — Chlorine breaks down ozone molecules.
• — increases the amount of UV rays reaching Earth’s surface
• Note: Do not allow credit for “chlorine” for the chemical symbol because this is the name of the
• element not the chemical symbol.
• Do not allow credit for “CL” for the chemical symbol because this is not the correct format for writing chemical symbols.
